ARVYS Proteins Inc. provides a full spectrum of protein biochemistry services – recombinant protein expression in bacterial, insect and mammalian cells, protein purification, refolding, assays and assay development, protein characterization, fermentation and endotoxin removal.
Outsource your protein biochemistry projects to ARVYS and enjoy superior results, team expertise and customer support after project completion.
Today we look at the publication from Humana Press. Series Editor John M. Walker School of Life and Medical Sciences University of Hertfordshire Hatfield, Hertfordshire, AL10 9AB, UK
Preface
Recombinant protein production in plants is becoming an increasingly attractive alternative to conventional production platforms due to promising cost and safety benefits.
Up-to-date scientific achievements from the world’s top researchers are conveniently arranged in the new addition of Recombinant Proteins from Plants as a collection of proto- cols for use with a variety of plant expression systems. Various aspects of production are covered including vector selection and cloning; product improvements for stability, glyco- sylation, and antibiotic-free selection; extraction and scale-up; and analysis of transgenic plants and their recombinant proteins.
This new edition of Recombinant Proteins from Plants is an ideal reference for those who are interested in plant molecular biology and molecular farming.
Abstract
Plastid transformation for the expression of recombinant proteins and entire enzymatic pathways has become a promising tool for plant biotechnology in the past decade. Several improvements of the technology have turned plant plastids into robust and dependable expression platforms for multiple high value compounds. In this chapter, we describe our current methodology based on Gateway® recombinant cloning, which we have adapted for plastid transformation. We describe the steps required for cloning, biolistic transformation, identication, and regeneration of transplastomic plant lines and Western blot analysis.
Areas that stand to be mentioned:
Fig. 1 Schematic representation of the construction of the plastid transformation vector. (a) The destination vector pDEST-CP is created by blunt end ligation of the Gateway® reading frame cassette (attR1-ccdB-CmR- attR2) into a suitable backbone vector containing the homologous regions necessary for integration into the chloroplast genome (INSL/INSR). (b) The BP reaction (attB×attP→attL+attR) is performed between the attB sites anking the goi sequence and the attP sites of pDONRTM221 resulting in the Entry vector pENTR- goi. (c) The LR reaction (attL × attR → attB + attP) between the pENTR-goi and the pDEST-CP yields the nal plastid transformation vector pEXP-goi. The pDONRTM221 is recreated as a by-product of the LR reaction. However, the OmniMaxTM E. coli cells that are transformed with this plasmid will not survive due to the lethal effects of the ccdB gene product [6]. Therefore, colonies should contain the pEXP-goi plasmid only. aadA spectinomycin resistance gene, Spec spectinomycin, INSL/INSR left/right insertion site, Am ampicillin, AmR ampicillin resistance gene, ccdB control of cell death gene, Cm chloramphenicol, CmR chloram- phenicol resistance gene, Km kanamycin, KmR kanamycin resistance gene, attB1/B2/P1/P2/L1/L2/R1/R2 Gateway® recombination sites
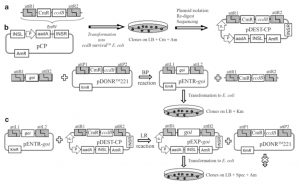
Fig. 2 Example of restriction digest pattern of putative pDEST-CP. DNA isolated from colonies grown on LB medium containing both ampicillin and chloramphenicol is digested with BamHI. (a) Schematic representa- tion of pDEST-CP with proper orientated Gateway® Rf; (b) Schematic representation of pDEST-CP with reverse oriented Gateway® Rf; (c) BamHI-digested putative pDEST-CP: Lane 2, 3 and 4 show the expected restriction pattern for correct insertion of the Rf into the vector backbone. Lane 1 shows the expected bands for reverse insertion of the Rf into the vector backbone. M Lambda DNA/Eco130I (StyI) Marker 16, Fermentas. aadA spectinomycin resistance gene, INSL/INSR left/right insertion site, AmR ampicillin resistance gene, ccdB control of cell death gene, CmR chloramphenicol resistance gene, KmR kanamycin resistance gene, attB1/ B2/P1/P2/L1/L2/R1/R2 Gateway® recombination sites, P promoter
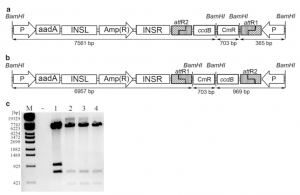
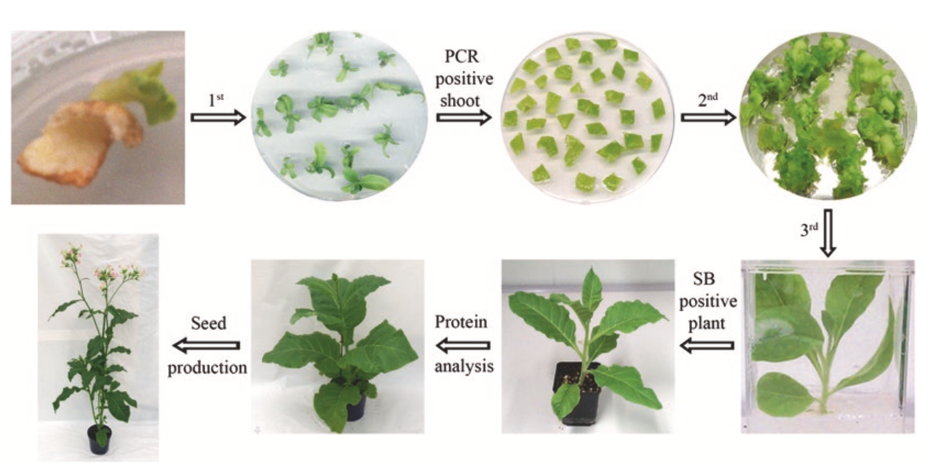
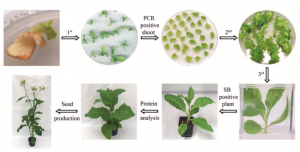
Fig. 3 Regeneration of transplastomic plant. In the rst round of regeneration the shoots emerging from the bombarded tissue cultivated on spectinomycin-containing RMOP medium are transferred to fresh medium and tested for transgene integration by PCR. PCR-positive shoots are subjected to a second round of regeneration by cutting them into small pieces and placing them on new spectinomycin-containing RMOP medium. Newly emerging shoots are rooted as a third round of regeneration and the homoplastomic state of the plant is veri- ed by Southern blot (SB). Homoplastomic plants are transferred to soil, protein analyses are carried out and seeds are produced
To buy the full journal, click here.
ARVYS Proteins Inc. provides a full spectrum of protein services to the life science, pharmaceutical and biotechnology communities. Our work experience encompasses almost every aspect of protein biochemistry allowing us to contribute to projects regardless of whether they are at early research or late development stages. We can be your partner in:
– generation and expression of recombinant proteins in bacterial, baculovirus and mammalian expression systems,
– large-scale fermentation,
– cell culture,
– purification of recombinant proteins, antibodies or naturally occurring proteins,
– refolding from inclusion bodies,
– improvement of protein stability,
– protein labeling with fluorescent, biotin and enzyme probes,
– endotoxin removal and testing for in vivo studies,
– protein characterization to monitor its integrity and functionality
“Don’t think clean thoughts with dirty enzymes” – is a famous saying of Efraim Racker, one of the founding fathers of modern biochemistry. We believe this philosophy should be extended to all proteins – purified and well-characterized preparations will save you time, effort and resources. Your confidence in protein reagents is the core focus of our business.