ARVYS Proteins Inc. provides a full spectrum of protein biochemistry services – recombinant protein expression in bacterial, insect and mammalian cells, protein purification, refolding, assays and assay development, protein characterization, fermentation and endotoxin removal.
Outsource your protein biochemistry projects to ARVYS and enjoy superior results, team expertise and customer support after project completion.
Affiliation – Stockholm Center for Biomembrane Research, Department of Biochemistry and Biophysics, Stockholm University, The Arrhenius Laboratories for Natural Sciences, Stockholm, SE-10691, Sweden.
Abstract
Membrane proteins control a large number of vital biological processes and are often medically important-not least as drug targets. However, membrane proteins are generally more difficult to work with than their globular counterparts, and as a consequence comparatively few high-resolution structures are available. In any membrane protein structure project, a lot of effort is usually spent on obtaining a pure and stable protein preparation. The process commonly involves the expression of several constructs and homologs, followed by extraction in various detergents. This is normally a time-consuming and highly iterative process since only one or a few conditions can be tested at a time. In this article, we describe a rapid screening protocol in a 96-well format that largely mimics standard membrane protein purification procedures, but eliminates the ultracentrifugation and membrane preparation steps. Moreover, we show that the results are robustly translatable to large-scale production of detergent-solubilized protein for structural studies. We have applied this protocol to 60 proteins from an E. coli membrane protein library, in order to find the optimal expression, solubilization and purification conditions for each protein. With guidance from the obtained screening data, we have also performed successful large-scale purifications of several of the proteins. The protocol provides a rapid, low cost solution to one of the major bottlenecks in structural biology, making membrane protein structures attainable even for the small laboratory.
Results:
We set out to design a protocol for rapid microscale solubilization and affinity purification in a 96‐well format, in order to be able to quickly evaluate whether a particular membrane protein is amenable to production for structural studies or not. For comparative purposes, we selected a battery of 16 commonly used detergents of varying size and head group chemistry, divided into five detergent families (Supporting Information Table SI). The screening protocol was engineered to be as fast and small‐scale as possible, while still simulating large‐scale preparation procedures closely; the latter to be able to identify conditions that allow for upscaling.
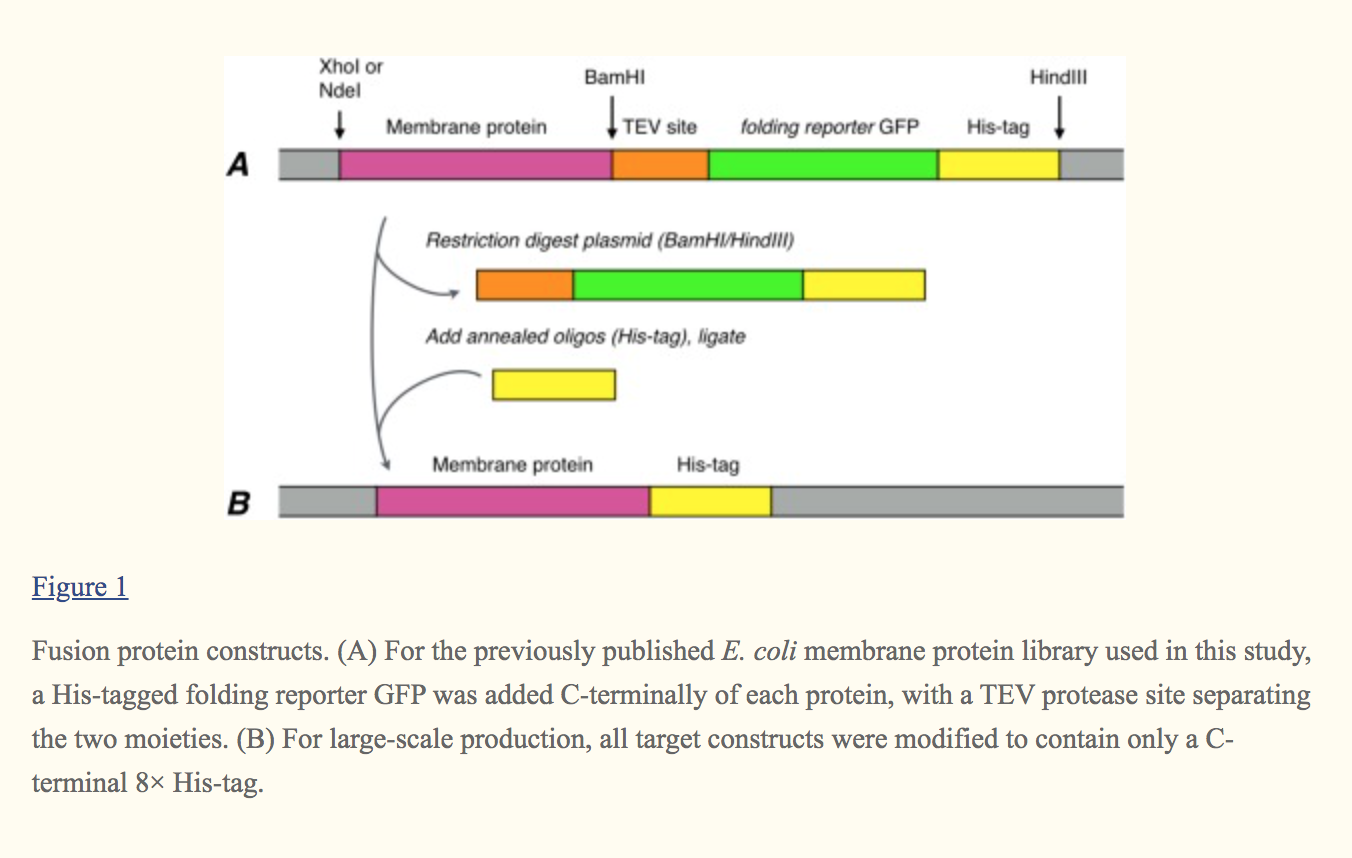
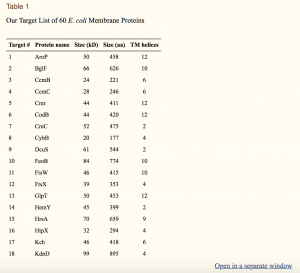
For the screening trials, 60 proteins were selected from a folding reporter GFP fusion membrane protein library where the membrane protein is followed by a TEV protease cleavage site, a C‐terminal folding reporter GFP molecule and a C‐terminal His‐tag [Fig. [Fig.11(A)].21 The proteins were chosen to cover a wide range of molecular weights and globular/transmembrane domain ratios (Table 1).
To buy the full journal, click here.
ARVYS Proteins Inc. provides a full spectrum of protein services to the life science, pharmaceutical and biotechnology communities. Our work experience encompasses almost every aspect of protein biochemistry allowing us to contribute to projects regardless of whether they are at early research or late development stages. We can be your partner in:
– generation and expression of recombinant proteins in bacterial, baculovirus and mammalian expression systems,
– large-scale fermentation,
– cell culture,
– purification of recombinant proteins, antibodies or naturally occurring proteins,
– refolding from inclusion bodies,
– improvement of protein stability,
– protein labeling with fluorescent, biotin and enzyme probes,
– endotoxin removal and testing for in vivo studies,
– protein characterization to monitor its integrity and functionality