In this series, we are highlighting some of our founder, Dr. Yelena Sheptovitsky’s, favorite manuscripts, publications, and news pieces all relating to the world of protein expression, life science, and CRO’s.
Today we look at the author manuscript from “Fusion Protein Linkers: Property, Design and Functionality” Adv Drug Deliv Rev. 2013 October 15; 65(10): 1357–1369 by Xiaoying Chen1, Jennica Zaro1, and Wei-Chiang Shen, School of Pharmacy, University of Southern California, Los Angeles, California 90089, USA.
ABSTRACT
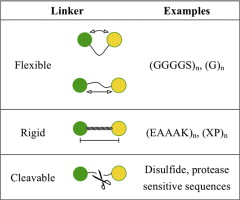
As an indispensable component of recombinant fusion proteins, linkers have shown increasing importance in the construction of stable, bioactive fusion proteins. This review covers the current knowledge of fusion protein linkers and summarizes examples for their design and application. The general properties of linkers derived from naturally-occurring multi-domain proteins can be considered as the foundation in linker design. Empirical linkers designed by researchers are generally classified into 3 categories according to their structures: flexible linkers, rigid linkers, and in vivo cleavable linkers. Besides the basic role in linking the functional domains together (as in flexible and rigid linkers) or releasing free functional domain in vivo (as in in vivo cleavable linkers), linkers may offer many other advantages for the production of fusion proteins, such as improving biological activity, increasing expression yield, and achieving desirable pharmacokinetic profiles.
One area that stands to be mentioned:
Linkers can improve folding and stability of fusion proteins
The flexible GS linker has been shown to improve folding and stability in several fusion protein examples. A first and very important application of the flexible GS linker is the construction of single-chain variable fragment (scFv), an antigen-binding fusion protein composed of antibody light-chain variable region (VL) tethered to heavy chain variable region (VH) via an oligopeptide linker [45]. A flexible linker (GGGGS)3 was designed by Huston et al. to construct a scFv, since its flexible structure could allow for the correct orientation of the VH and VL domains, and would not interfere with the folding of the protein domains [46]. The length of the linker was adjusted according to the distance between the C-terminus of the VH domain and the N-terminus of the VL domain under its natural orientation (3.5 nm). The length of the (GGGGS)3 linker was calculated to be about 5.7 nm, and was expected to bridge the VH and VL domains [46]. This (GGGGS)3 linker was demonstrated to be suitable for constructing scFv due to its high flexibility, and has since been applied to many other scFvs [47-49].
Another type of flexible linker, (Gly)n, has also been shown to improve folding of fusion proteins [30]. The insertion of the (Gly)8 linker between a Myc epitope tag and the protein of interest (Est2p) greatly improved the functionality of epitope-tagged Est2p. The improvement was attributed to the correct folding of the epitope-tagged protein after linker insertion, as well as the reduced steric hindrance between functional domains.
In addition to flexible linkers, helical linkers can also improve fusion protein folding and stability. For instance, in the development of a virus coat protein-fusion protein, only peptides shorter than 20 amino acids could be expressed onto the coat protein of plant rod- shape viruses without preventing the assembly of functional virions [50]. Dramatically, with the aid of a 15-amino acid linker (EAAAK)3, a protein A fragment of 133 amino acids could be displayed onto the viral coat protein and the resultant fusion proteins could form functional virions [51]. By providing enough distance between protein A and the coat protein, the rigid helical linker permitted correct folding of the fusion protein, and allowed for the correct assembly of virus particles.
The length and structure of the linkers can also control the distance between functional domains and affect the stability of the fusion protein [34]. The α-helical linkers (EAAAK)n (n=1-3) as well as a flexible linker were applied to construct bifunctional fusions of β- glucanase and xylanase enzymes. The stability and catalytic activity of the resultant fusion proteins were significantly improved after linker insertion [52]. In addition, the thermal stability of β-glucanase in the fusion proteins was improved as the length of the helical linkers (controlled by copy number “n”) increased. Compared to flexible linkers, helical linkers appeared to be more efficient in increasing the thermal stability. These improvements were likely due to the rigid structure of the α-helical linker that might provide enough space for protein domains to fold and function independently. These studies indicated that the insertion of a linker with proper structure and length could ultimately improve the stability and bioactivity of functional moieties.
To read the full manuscript, click here.
ARVYS Proteins, Inc. is a leader in the space of Antibody Development & Production. From generation of antigen to characterization of antibodies ARVYS Proteins provides protein science services for critical projects.



