Endotoxins are toxic protoplasms that are released when bacteria grow, die, and disintegrate. These endotoxins are lipopolysaccharides, which reside in the outer membrane of gram-negative bacteria. The gram-negative cell wall is composed of a thin, inner layer of peptidoglycan and an outer membrane of phospholipids, lipopolysaccharides, and lipoprotein.
| PROPERTY | ENDOTOXIN |
| Chemical nature | Lipopolysaccharide |
| Relationship to cell | Outer membrane |
| Size | 10 kilodalton |
| Isoelectric point | 2.5 |
| Denatured by boiling | No |
| Antigenic | Yes |
| Form toxoid | No |
| Potency | >100 micrograms |
| Specificity | Low degree |
| Enzymatic activity | No |
| Pyrogenicity | Yes |
The lipopolysaccharides, or endotoxins, are made up of O-antigens, a core polysaccharide, and lipid A, which is made up of glucosamine and fatty acids and is responsible for the toxic effects. The toxins are continuously shed from the outer membrane of bacteria, and released into the environment. When proteins are purified from E. coli, the unwanted endotoxin by-products must be removed from the final product for manufacturing processes and in vivo applications.
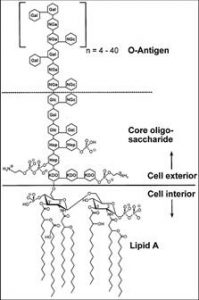
The toxicity of endotoxins makes their removal an important step for protein application in several biological assays and for safe release of product, as required by the FDA. The endotoxin limit for injections can be calculated by dividing the minimum pyrogenic dose of endotoxin per kilogram by the maximum dose of product per kilogram per hour.
| TYPE | INTRATHECAL | ENDOTOXIN LIMIT |
| Injectable drug | Yes | .2 endotoxin unit per kilogram |
| Injectable drug | No | 5 endotoxin units per kilogram |
| Medical device | Yes | .06 endotoxin units per milliliter |
| Medical device | No | .5 endotoxin units per milliliter |
| Radiopharmaceuticals | Yes | 14 endotoxin units per volume |
| Radiopharmaceuticals | No | 175 endotoxin units per volume |
| Sterile water | – | .25 endotoxin units per millimeter |
| Assay | – | Positive product controls recovery >50% |
Check out http://www.qimedical.com/links/Endotoxin_Limits_Document_2011.pdf to compare various endotoxin limits for common injectables set by the United States Pharmacopoeia
Increases in the production of cytokine, prostaglandin, acid phosphatase, fibrinolytic inhibitor, collagenase production, nerve growth factor, factor B, polypeptides, platelet activating factor, adhesion inhibitor, adhesion molecule-1 and procoagulant can result from endotoxin consumption, and thus can cause multiple responses which vary between animals and humans.
| ANIMALS | HUMANS |
| Fever | Fever |
| Decrease in blood pressure | Decrease in blood pressure |
| Disseminated intravascular coagulation | Intravascular coagulation |
| Hypotension | Hypotension |
| Shock | Shock |
| Change in instincts for survival | Inflammation |
| Severe blood flow changes | Septic shock |
| Changes in metabolic hormones | Tissue injury |
| Poor growth | Vasodilation |
| Changes in white blood cell counts | Fibrinolysis |
| Death | Hemorrhaging |
Normally, when animals are injected with even small doses of endotoxin, most experience a latent period, physiological distress such as diarrhea, prostration, and shock, and then finally death. They vary in their susceptibility to endotoxin. With humans, it’s more complex. The endotoxins bind to a lipid binding protein in the serum and are transferred to the CD14 receptor on the cell membrane. It is then transferred to the MD2 protein, which is associated with the TLR$, which triggers a cascade of signals for cells to secrete pro-inflammatory molecules, which can lead to various responses.
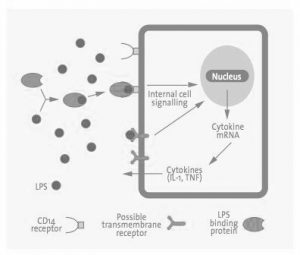
Endotoxins are a natural part of gram-negative bacteria such as Acinetobacter, Actinobacillus, Bordetella, Brucella, Campylobacter, Cyanobacteria, Enterobacter, Erwinia, Escherichia coli, Franciscella, Helicobacter, Hemophilus, Klebsiella, Legionella, Moraxella, Neisseria, Pasteurella, Proteus, Pseudomonas, Salmonella, Serratia, Shigella, Treponema, Vibrio, and Yersinia. Any samples that are derived from gram-negative bacterial cultures have a high presence of endotoxins. In addition, adventitious endotoxins can be found everywhere bacteria had have a minute presence including dust, dirt, and air. In laboratory environment endotoxins can come from water, sera, media, reagents, buffers, plasticware and glassware. Since endotoxins have high stability in both high temperature and high pH, they cannot be removed by sterilization. Due to its wide-spread presence and low thresholds for pyrogenic reaction, it is essential to remove endotoxins from samples that are destined for in vivo use, from therapeutics to ensure the health of the consumers.
Endotoxin removal methods for protein solutions are usually based on affinity, ion-exchange chromatographies, or detergent phase partitioning. Multiple cycles must be done to completely remove endotoxins and make products non-pyrogenic. (www.arvysproteins.com).
- Affinity Chromatography: Principles and Methods, AC ed.; Amersham Pharmacia Biotech: Upsala, Sweden. 2001.
- Bordier C. “Phase separation of integral membrane proteins in Triton X-114 solution.” The Journal of Biological Chemistry. Feb 1981, 256:1604-1607.
- Cohen J and Davis B. “Endotoxin Removal Devices for the Treatment of Sepsis and Septic Shock.” The Lancet Infectious Disease. 2011 Jan;11(1):65-71.
- Guadagni G, Perego A, Shoji H, and Tani T. “Extracorporeal Removal of Endotoxin: The Polymyxin B-immobilized Fiber Cartridge.” Contributions to Nephrology. 2010;167:35-44.




